Chapter 2: Separating and purifying substances
Separating heterogeneous mixtures
- Decantation- This is the process of removing a liquid from a solid (Like sand and water) which has settled (sedimented) or form an immiscible heavier liquid (like oil and water) by carefully pouring. It is important you learn this definition, asked in paper 6.
- Filtration- This is where an insoluble material is collected on filter paper- this is the residue. The liquid phase obtained is called the filtrate. It is useful since both phases can be obtained.

- Centrifugation- The separation of an insoluble solid from a liquid in a test tube by rapid spinning during which the solid collects at the bottom of the sample. The liquid then can be decanted carefully.

- Separating Funnel- This is used to separate immiscible liquids like oil and water. The tap is opened to let the water out first. Then close tap when water is finished and change beaker and empty oil. Hence both liquids are separated.
- Magnetic Properties- Suppose we have a mixture of iron and plastic. We can hence separate iron from the plastic by using a magnet to attract all the iron pieces from the plastic.
- Solubility- If we have a mixture of salt and sand. We can take water which dissolves the salt and not the sand therefore separating the mixture.
- Sublimation- If we have ammonium chloride and sodium chloride (Salt), ammonium chloride sublimes while salt doesn't therefore the mixture is separated.
Separation of Homogeneous Mixtures
- Evaporation and Crystallization- Used to separated solid in liquid mixtures (like salt and water) Evaporation is used if you want the powder of solid like salt. Evaporation is done by heated the solution on a evaporating dish, letting the liquid evaporate and solid is left. In crystallization the solution is concentrated by evaporating only some of the liquid in a water bath. When small crystals form on glass rod when dipped, it goes for cooling and crystals of solid is formed. The crystals are then filtered off and dried in oven or using filter paper (like tissue paper)
 Evaporation
Evaporation
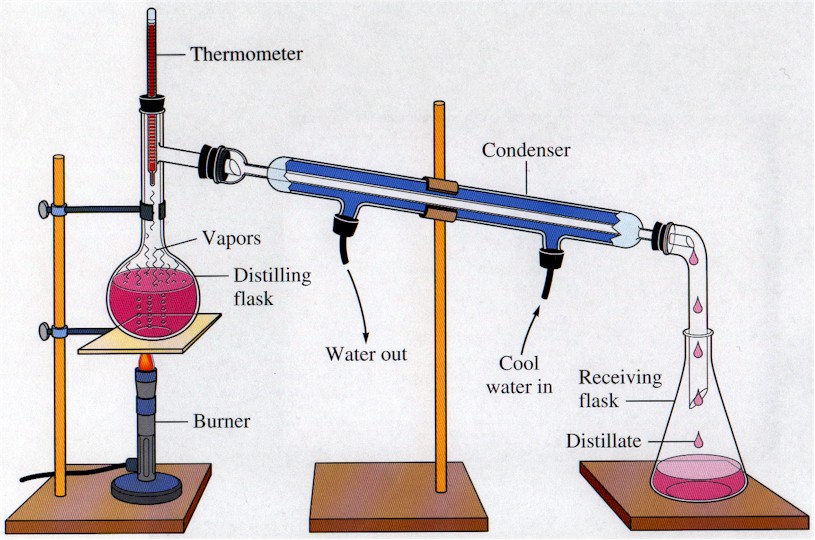
- Distillation- Used to obtain liquid from solution. Liquid is first evaporated and then condensed in the condenser. The liquid obtained is known as distillate.
- Fractional Distillation- This is often asked in the exam so pay attention. This is used to separate a mixture of liquids. The liquids have different boiling points hence can be separated. Suppose we have ethanol (alcohol) and water. Ethanol has a lower boiling point (78C) than water (100C) therefore gets boiled first. The water vapor keeps condenses in the fractional column since its below its boiling point.

- Chromatography- This is used to separate a mixture of solids dissolved in a liquid like coloured dyes by differences in their solubility in a solvent. Please learn the procedure for chromatography since it is frequently asked in most of the papers it is extremely important (bold)
- First draw a line on the chromatography paper (about 1cm form the bottom) with pencil. This is called the base line.
- Place a drop of the solution on the base line.
- Place the chromatography paper in a solvent and the solvent shouldn't cross the base line.
- As solvent moves up the line the dyes with it start to separate into different colours.
- If the solution is invisible like amino acids, use locating agent to locate the spots and warm the paper in the oven then spots will reveal.
- Calculate Rf value using the formula = Distance moved by substance/ Distance moved by solvent front. The solvent front is where the solvent stops. The identity of a substance in the dye can be checked by comparing its Rf value to that of a sample we know is pure. Suppose Rf value of an amino acid is 0.31 (random) and the Rf value of a spot on the chromatography paper is 0.31 therefore the solution has an amino acid.
- One spot means that the solution is pure.

Solubility
- Solution is made of solute (solid dissolved) and solvent ( the liquid in which the solid dissolves in).
- Solubility of solids in liquids increase with temperature. At a fixed temperature there will come a point where no more sold will dissolve in the solvent. This means that the solution is saturated. Temperature has to increase then to dissolve more of the solid.
- Solubility of gases in liquid decreases with an increase in temperature.
- Experiment to find solubility of solid in liquid (asked in p6 once so I recommend you to learn the procedure)
- Take known mass of the solid using balance and take 100 cm3 of water using burette in beaker.
- Heat the water ti the required temperature like 30C
- Add the solid to the liquid and stir and keep adding till no more solid will dissolve in liquid and solution becomes saturated.
- Filter out excess solid that didn't dissolve in liquid.
- Then evaporate the liquid off and solid that dissolved remains.
- Measure the mass of solid that dissolved in the liquid obtained in the evaporation.
- Calculate solubility of solid at the fixed temperature using mass of solid dissolved (say in grams) divided by volume of liquid used (100cm3 in this example). Units will be g/100cm3 then.


 Evaporation
Evaporation



No comments:
Post a Comment