Important definitions-
Atom- The smallest particle of an element that can take part in a chemical reaction. All atoms of the same element have the same number of protons in the nucleus.Pure substances can be of two types-
- Element- Substances that cannot be chemically broken down into simpler substances. An element cannot be decomposed by passing electric current through it.
- Compound- Pure substances made from two or more elements chemically combined together in fixed proportions.
Mixtures can be of two types-
- Homogeneous
- Heterogeneous (explained in the previous blog)
Difference between Mixtures and compounds
It is really important to understand the difference since most students don't understand the difference.
No
|
Mixtures
|
Compounds
|
1
2
4
|
Substances simply mixed together, no reaction takes place
Composition of mixture can be varied like in air the carbon dioxide
level could rise and fall, no fixed value
Properties of the substances present remain the same.
Substances in the mixture can be separated by physical means like
filtration and distillation
|
Substances chemically react together to form a new compound
Composition of new compound remains the same.
Properties of the new compound formed are different form the elements
it is made of.
Compound cannot be easily separated into its elements
|
Kinetic Theory (the Dalton theory not required by syllabus,
- All matter is made up of small particles
- The particles are moving all the time. The higher the temperature the higher the average energy and speed of particles.
- Heavier particles move more slowly than lighter particles at the same temperature. Then take a look at http://igcsechemistryrevision.blogspot.in/2013/03/chapter-2-states-of-matter-and.html for application of kinetic theory in changes in physical states of matter.
Diffusion
- This is the process by which different substances mix as a result of the random motion of their particles. This is the movement of particles from a region where they are in a higher concentration to region of lower concentration
- This eventually leads to the particles spreading out. to make the concentration same throughout.
- Diffusion only takes place in fluids (liquids and gases)
- The lower the molecular mass the faster the particle will diffuse. EG- Hydrogen gas has molecular mass of 2 (H2 =1*2=2) is more likely to diffuse faster than chlorine gas with molecular mass of 71 (Cl2 =35.5*2=71) since it is lighter.
Structure of Atom
- Atoms have three sub-atomic particles- proton, neutron and electron.
Sub atomic Particle
|
Relative Mass
|
Relative Charge
|
Location in atom
|
Proton
|
1
|
+1
|
In nucleus
|
Neutron
|
1
|
0
|
In nucleus
|
Electron
|
1/1840
|
-1
|
Outside nucleus in orbits
|
- Atomic number = Number of Proton
- Atomic Mass = Number of protons+ Number of neutrons=nucleons
- Number of Electrons = Number of protons = Atomic Number
- Number of neutrons = Mass number-Atomic number=nucleons-protons
- Isotopes- Atoms of the same element with different mass number. They have the same number of protons and electrons but different number of neutrons.They have the same chemical properties since they have the same number of electrons but only physical properties differ. Some isotopes maybe radioactive known as radio-isotopes.(Extremely important to remember this definition and properties of isotopes)
Radioactivity
- This is the spontaneous decay of unstable radio-isotopes. It is unaffected by temperature or whether the isotope is part of a compound or present as the free element, it is a totally random process.
- There are three types radioactivity
- Alpha Radiation
- Beta Radiation
- Gamma Radiation (no mass)
Radiation
|
Isotopes involved
|
Nature
|
Distance travelled in air
|
Penetration
Paper Thin Thick
Al
Lead
|
||
Alpha Radiation
|
Atomic Number >83
|
Alpha Particles
Helium He42 (double positive charge, no
electrons)
|
A few
|
No
|
No
|
No
|
Beta Radiation
|
Atomic Number ≤ 83
|
Beta Particles (electrons single –ve charge)
|
A few
|
Yes
|
No
|
No
|
Gamma Radiation
|
Only occurs with one of the other forms of radiation
|
Electromagnetic Radiation
|
Many
|
Yes
|
Yes
|
No
|
Alpha and beta radiation knocks out electrons out of atoms (ionization) to produce positive ions.
Uses of Radioactivity (Important to learn these uses, can be asked )
Industrial
- Radioactive Dating which measures age of an object.
- Monitors the level of filling in containers
- Checks thickness of sheets of metal or paper
- Detects leaks in gas or oil pipes
- To kill cancer cells using gamma radiation from cobalt-60
- To sterilize medical instruments, dressings and syringes by gamma radiation to kill bacteria.
- Food treatment to kill bacteria, mould and yeast by gamma radiation
Electron arrangements in atoms
- Electrons in atoms are arranged in an organised way in different shells
- These electron shells have different electron shells are at different distances from the nucleus of the atom
- Electrons are placed in the shells closest to the nucleus first,and each shell has a maximum number of electrons it can contain. This could be calculated using the formula 2n2 where n is the shell number. For example shell 1 can hold 2 electrons at maximum and shell 2 can hold maximum 8 electrons.
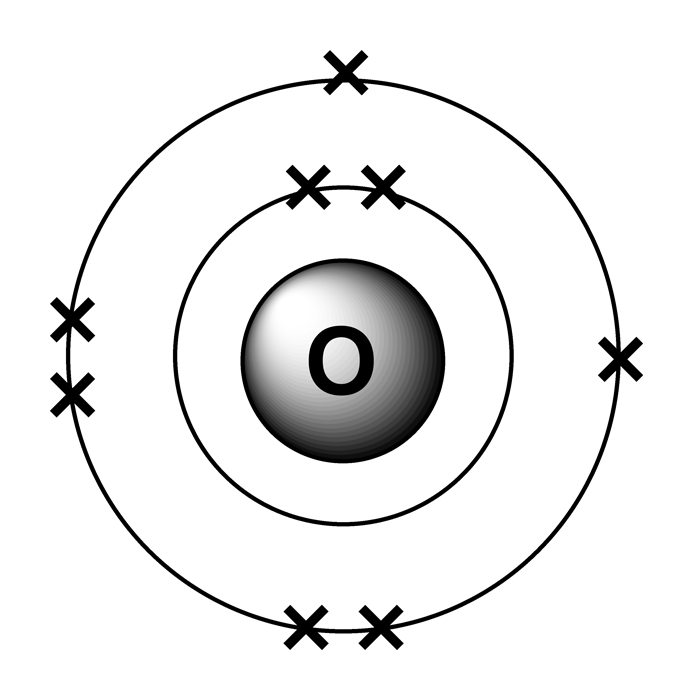
- Noble gas arrangement is when the outershell is filled with maximum electrons therefore is unreactive since its already stable. These elements are known as noble gases like helium has 2 electrons in outershell and the first shell can only hold 2 electrons not more than that therefore is stable.
- The number of electrons in the outershell play a very important role in deciding the chemistry of the element.
- Atoms gain or lose electrons or share electrons to attain noble gas arrangement. Only the outershell electrons are involved in bonding.Will be discussed later. But remember this, commonly asked question why atoms do this? To attain noble gas arrangement to be stable.
No comments:
Post a Comment